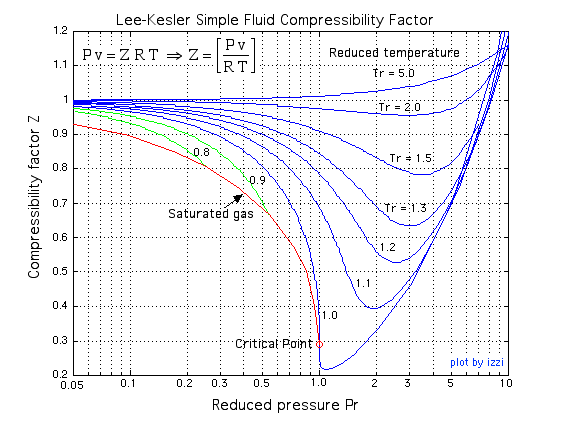
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
At Critical Temperature-pressure and volume - The compressibility Factor -Z- Is

Compressibility factor Z - Gaseous State
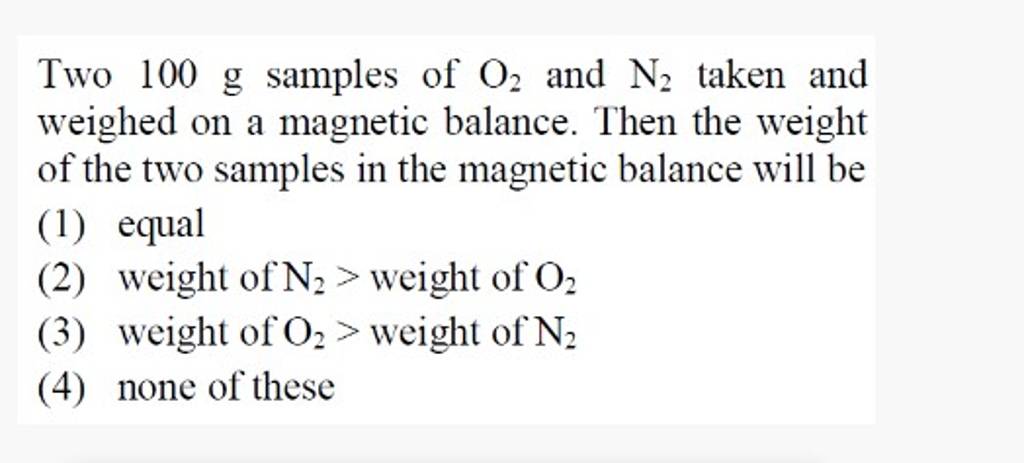
Two 100 g samples of O2 and N2 taken and weighed on a magnetic balance

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

SOLVED: at critical temperature, pressure and volume the compressibility factor z is?
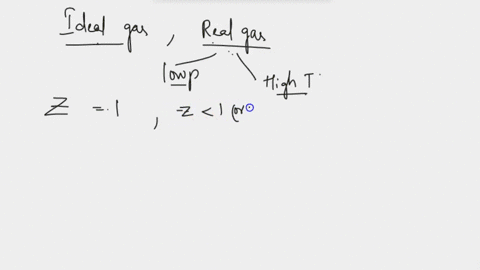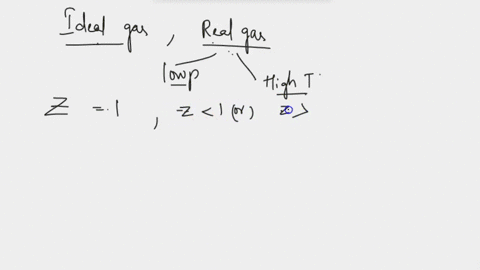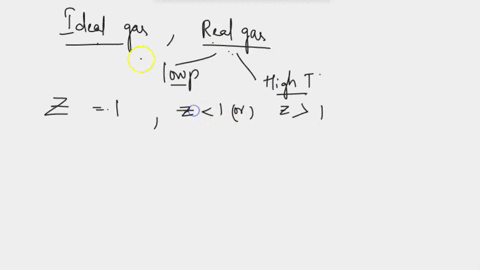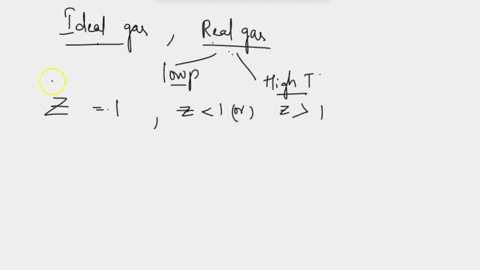
SOLVED: at Boyles temperature , compressibility factor Z of a real gas is

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02
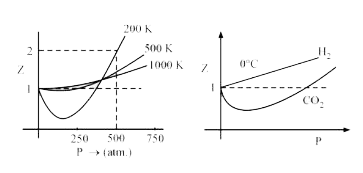
At Boyle's temperature, for a considerable range of pressure the value

At critical temperature, pressure and volume. the compressibility factor z is

Carbon dioxide compressibility factor determination using a robust intelligent method - ScienceDirect

Please answer ques no 22 EXERCISE that Oz as AOove O) aitk* - Chemistry - Electrochemistry - 13513747

If excluded volume is taken as zero, compressiblity factor Z is

Ch2, Lesson E, Page 9 - Generalized Compressibility Chart

At critical temperature, pressure and volume. the compressibility factor z is