Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2
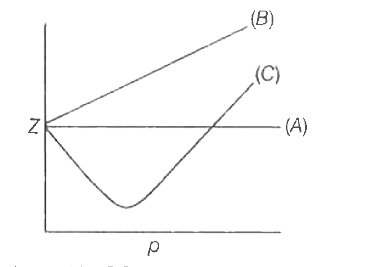
Telugu] The variation of compressibility factor (Z) with pressure (p

01 Gaseous State#### PDF, PDF, Gases

Gaseous State : Vander Waal Gas Equation - The Chemistry Guru
The given graph represent the variations of compressibility factor (z) = pV/ nRT versus p, - Sarthaks eConnect
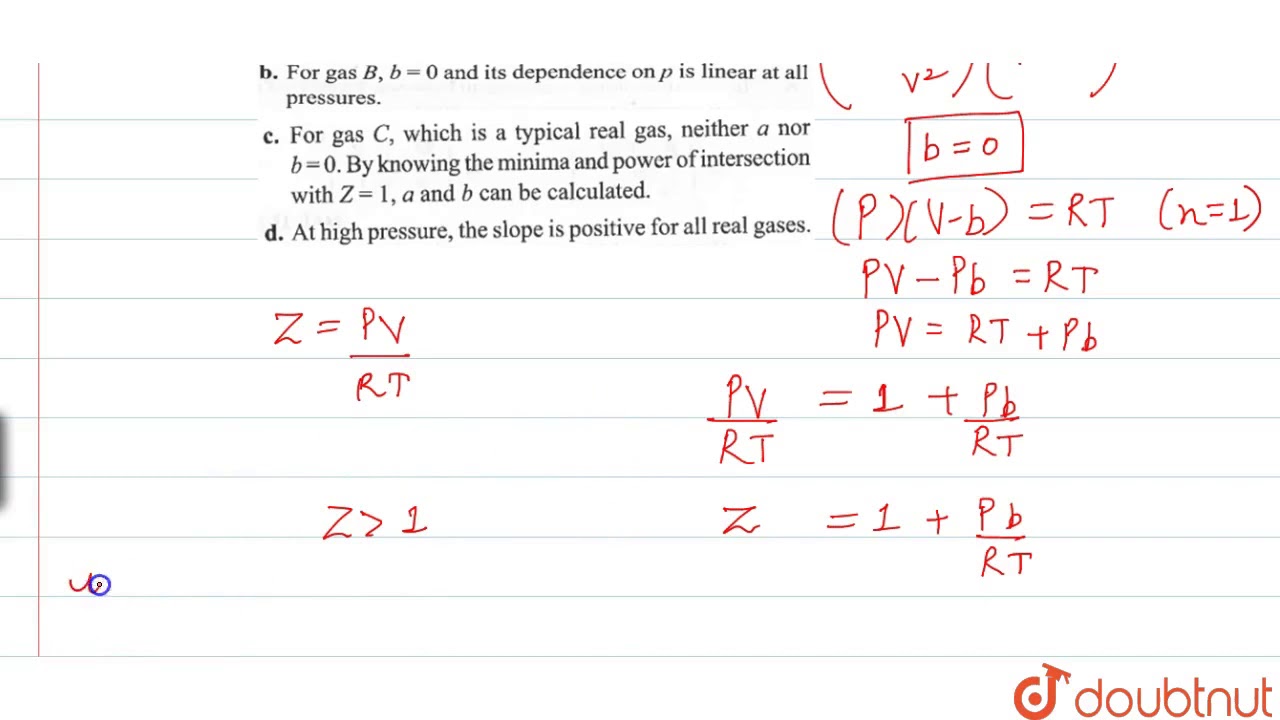
The given graph represents the variations of compressibility
Why does gas liquefy at high pressure? Even at high-pressure

PDF) Petroleum and natural gas production engineering

Determine Compressibility of Gases
What is the significance of the curve part in Z vs. P graph of compressibility of a gas? - Quora






