A new catalyst can make ethanol out of carbon dioxide
Scientists from Argonne National Laboratory, the University of Chicago’s Pritzker School of Molecular Engineering and Northern Illinois University have helped discover a new electrocatalyst that can consistently convert carbon dioxide and water into ethanol. That means the carbon dioxide emitted from industrial processes—such as fossil fuel or alcohol fermentation plants—can be turned into a valuable commodity at reasonable cost.

Catalysts, Free Full-Text

Proposed mechanism for carbon dioxide electroreduction. a The pathway

Chemistry University of Chicago News

staff University of Chicago News

Argonne National Laboratory
Carbon dioxide converted to ethylene -- the

Carbon Dioxide Conversion to Methanol: Opportunities and Fundamental Challenges

Science & Medicine University of Chicago News

New copper catalyst could close the carbon cycle, making ethanol from atmospheric CO2

Solved ( 2 and 3 ) Equal to two problems. To create
Can we build a device that converts carbon dioxide to oxygen? If yes, then How? - Quora

Will stored hydrogen (created during high wind/low demand periods in the UK) eventually replace the base load, rendering nuclear power superfluous? - Quora
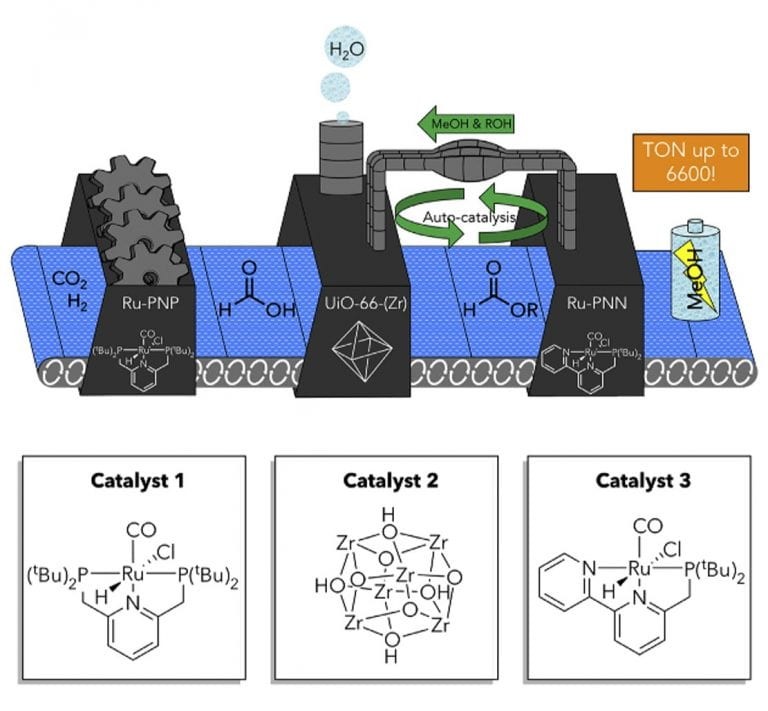
Tandem catalytic system efficiently converts carbon dioxide into methanol
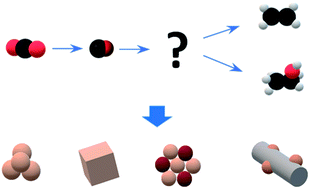
Electrochemical CO2 reduction to ethanol: from mechanistic understanding to catalyst design - Journal of Materials Chemistry A (RSC Publishing)