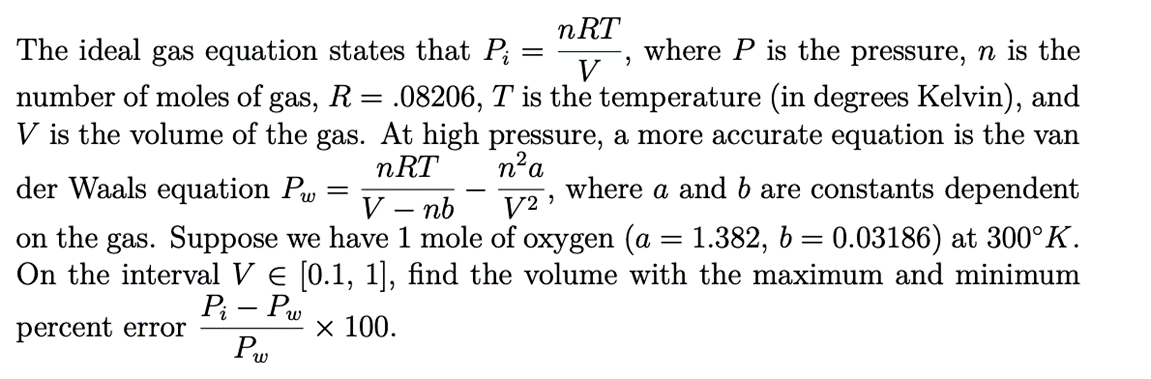

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through

An ideal gas has initial volume V and pressure p. If the volume of

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas
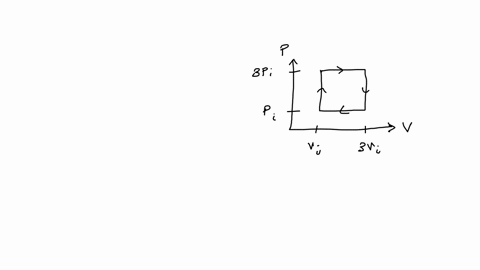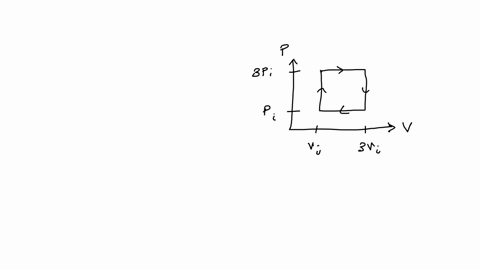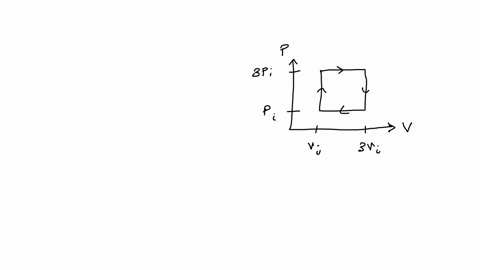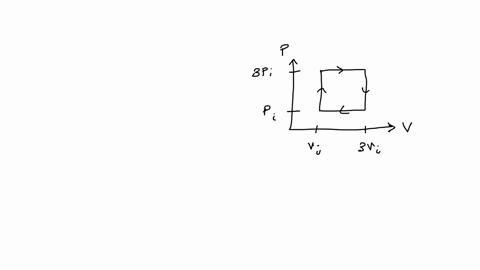
SOLVED: An ideal gas initially at Pi' Vi' and T; is taken through
Two moles of an ideal gas is compressed isothermally and
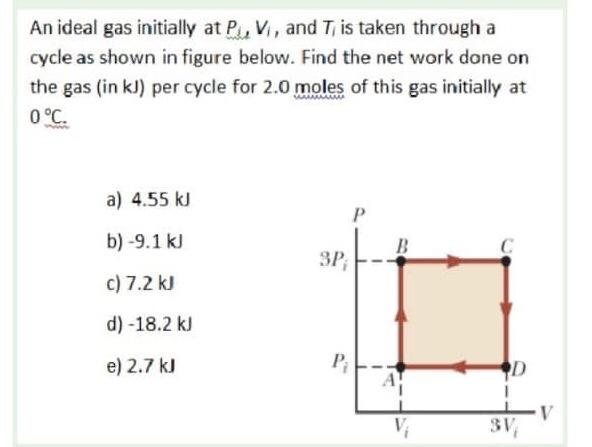
Solved An ideal gas initially at PJ, V, and Ti is taken

SOLVED: An ideal gas initially at Pi, Vi, and Ti is taken through

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle
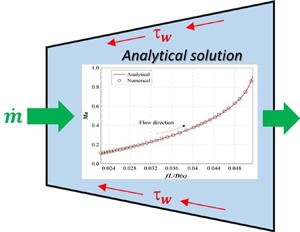
Exact solutions for quasi-one-dimensional compressible viscous

In the given figure an ideal gas changes its state from `A` to

The pressure volume work for an ideal gas can be calculated by
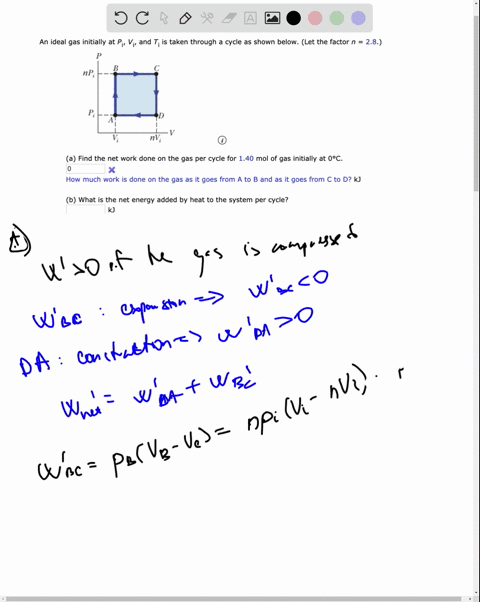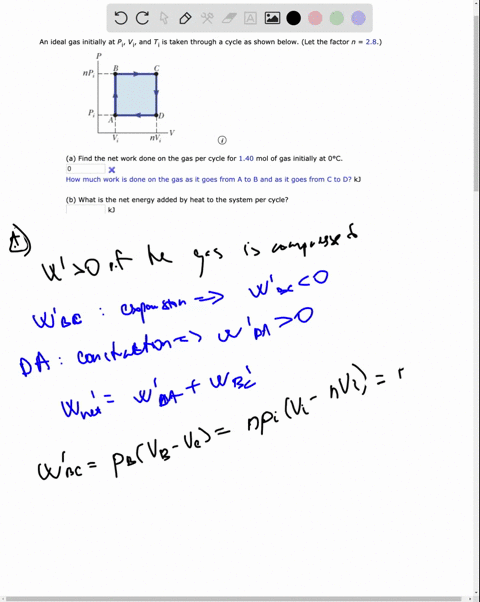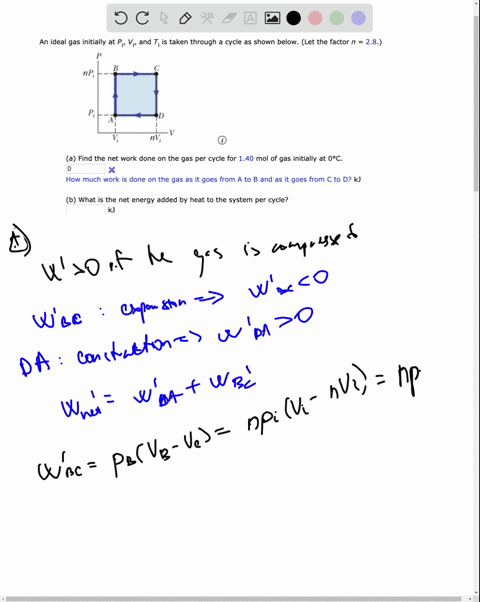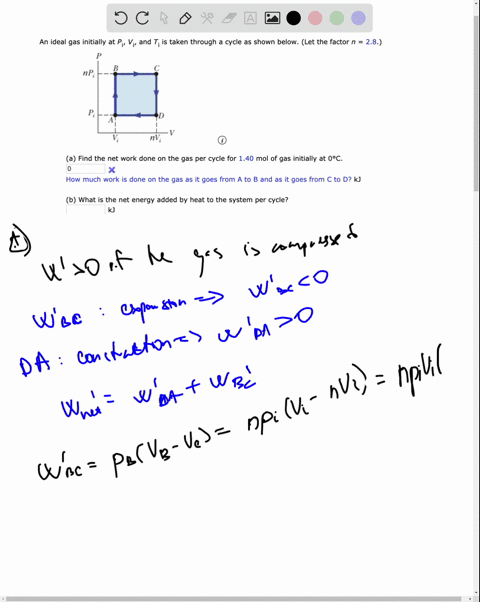
SOLVED: An ideal gas initially at Pi' Vi' and Ti is taken through

Gas Definition, State of Matter, Properties, Structure, & Facts

An ideal gas is taken through a process in which pressure and