What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?

Description
I found an increase of 3100J Have a look

SOLVED: What is the change in internal energy of a system if the

PDF) Theory & Problem of Heat Transfer

Solved Be sure to answer all parts. What is the change in

A system absorbs 50 kJ heat and does 20 kJ of work. What is the
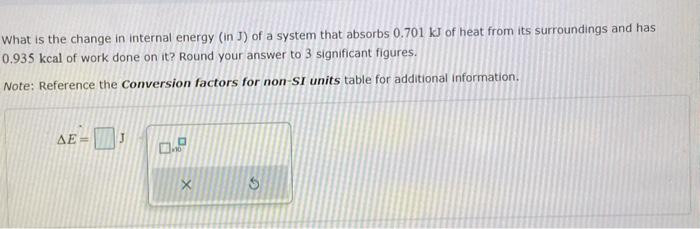
Solved What is the change in internal energy (in J) of a

Calculate the internal energy change for each of the following process
How to calculate ΔE when the system absorbs 250 J of heat energy

PDF) Useful conversion factors
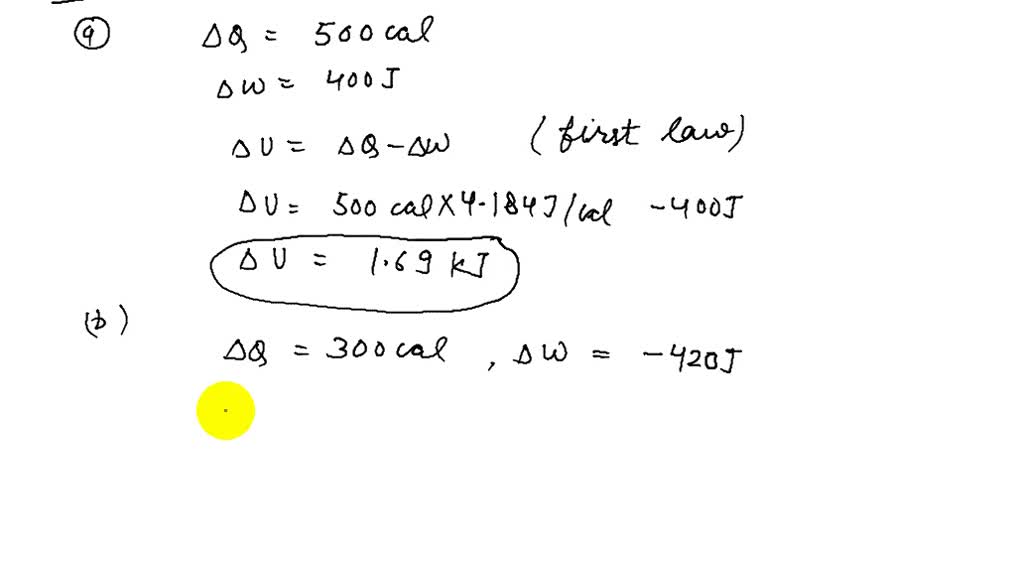
⏩SOLVED:In each of the following situations, find the change in
Related products
You may also like
Cartel: Ordenar la ropa de invierno y verano (teacher made)

Ilfioreemio Butt Lifting Workout Leggings for Women, Scrunch Butt

⚾️🔷🔴 I design a new Atlanta Braves jersey after every series win this season: “Peaches n' Cream” : r/Braves

Dermdoc Ceramides, Squalene & Vitamin E Deep Moisturizing Creme Body Lotion (200 ml)
$ 25.99USD
Score 4.5(129)
In stock
Continue to book
You may also like

Cartel: Ordenar la ropa de invierno y verano (teacher made)

Ilfioreemio Butt Lifting Workout Leggings for Women, Scrunch Butt

⚾️🔷🔴 I design a new Atlanta Braves jersey after every series win this season: “Peaches n' Cream” : r/Braves

Dermdoc Ceramides, Squalene & Vitamin E Deep Moisturizing Creme Body Lotion (200 ml)
$ 25.99USD
Score 4.5(129)
In stock
Continue to book
©2018-2024, farmersprotest.de, Inc. or its affiliates