What is the mass of glucose required to produce 44g of C{O_{2'}} on complete combustion?30g45g60g22g

Click here:point_up_2:to get an answer to your question :writing_hand:what is the mass of glucose required to produce 44g of co2 on complete
Click here👆to get an answer to your question ✍️ What is the mass of glucose required to produce 44g of C-O-2- on complete combustion-30g45g60g22g

What is the mass of glucose required to produce 44g of Co2 on complete combustion

Determine the mass of CO2 produced by burning enough of each fuel

Solved 1. The combustion of sugar produces carbon dioxide

What mass of glucose is required to produce 88 g of CO2 on complete combustion?

Solved -. The atomic mass of oxygen (O2) is 16.00 g/mol.

Solved For the following reaction, 9.40 grams of glucose
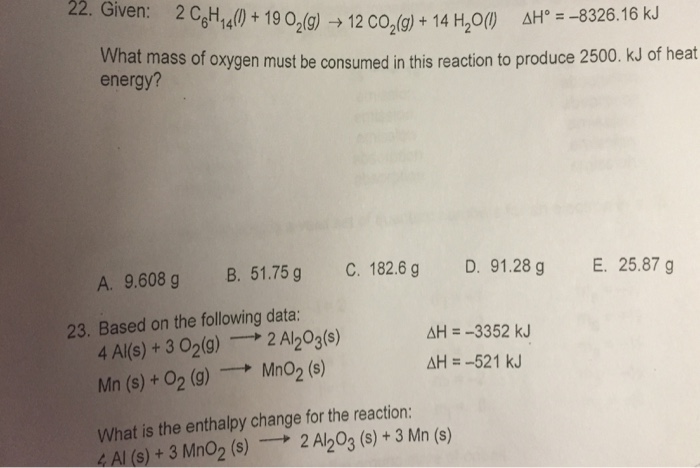
Solved 22 Given: 2 C, H140+19 C2 2 G c6H14() + 1902(g) → 12

what is the mass of glucose required to produce 44grams of CO2 on complete combustion - 10ijyqww

What is the mass of glucose required to produce 44 g of Co2 on complete combustion?1) 30 g(2) 45 g3) 60 g(4)

Solved Glucose, also known as blood sugar, has the

What is the mass of glucose required to produce 44 g of CO(2), on comp
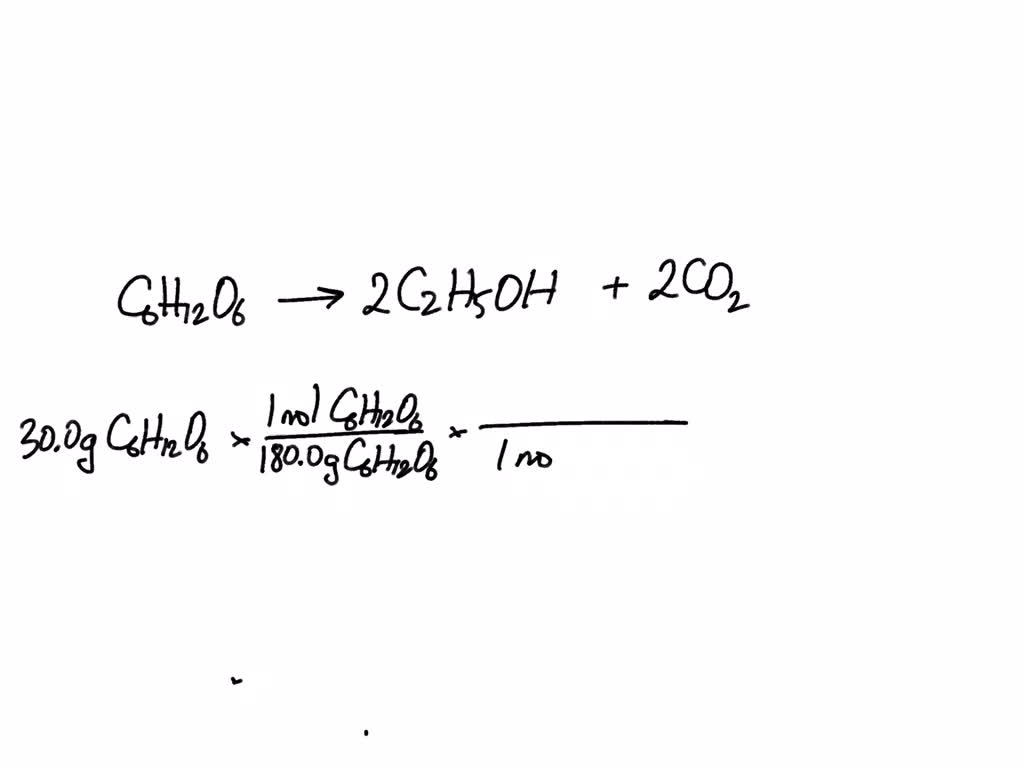
SOLVED: (ii) Calculate the maximum mass of ethanol that could be obtained from 30.0g of glucose
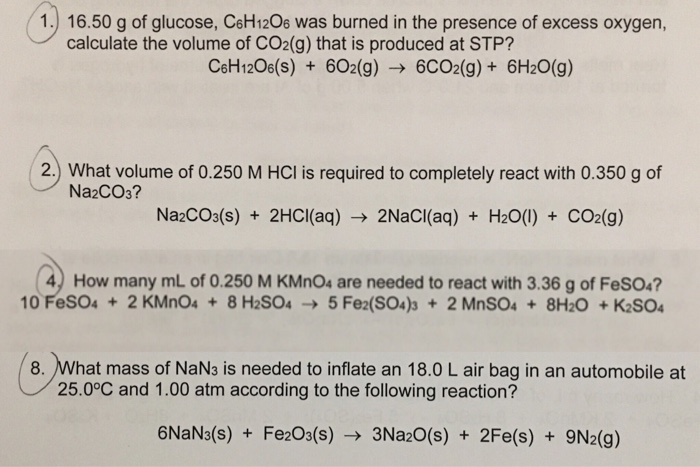
Solved 1.) 16.50 g of glucose, CsH120s was burned in the

Determine the mass of CO2 produced by burning enough of each fuel
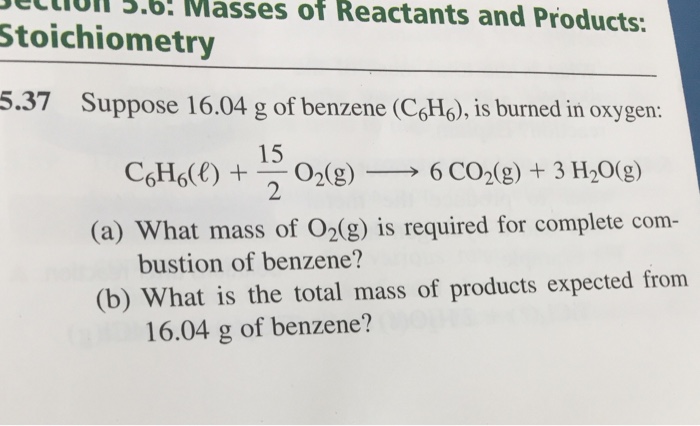
Solved Suppose 16.04 g of benzene (C_6 H_6), is burned in