The Cottrell Experiment and Diffusion Limitation 3/3

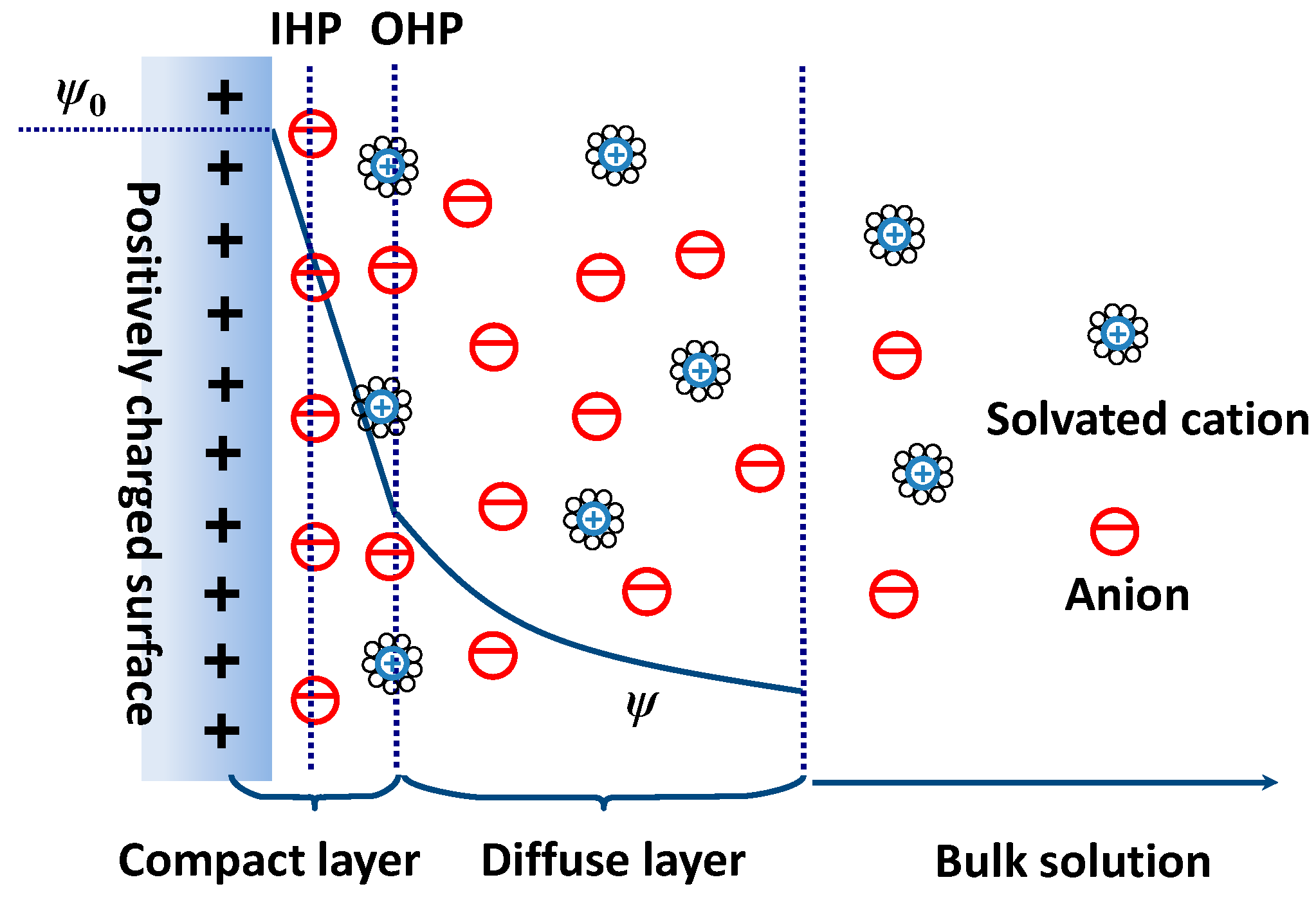
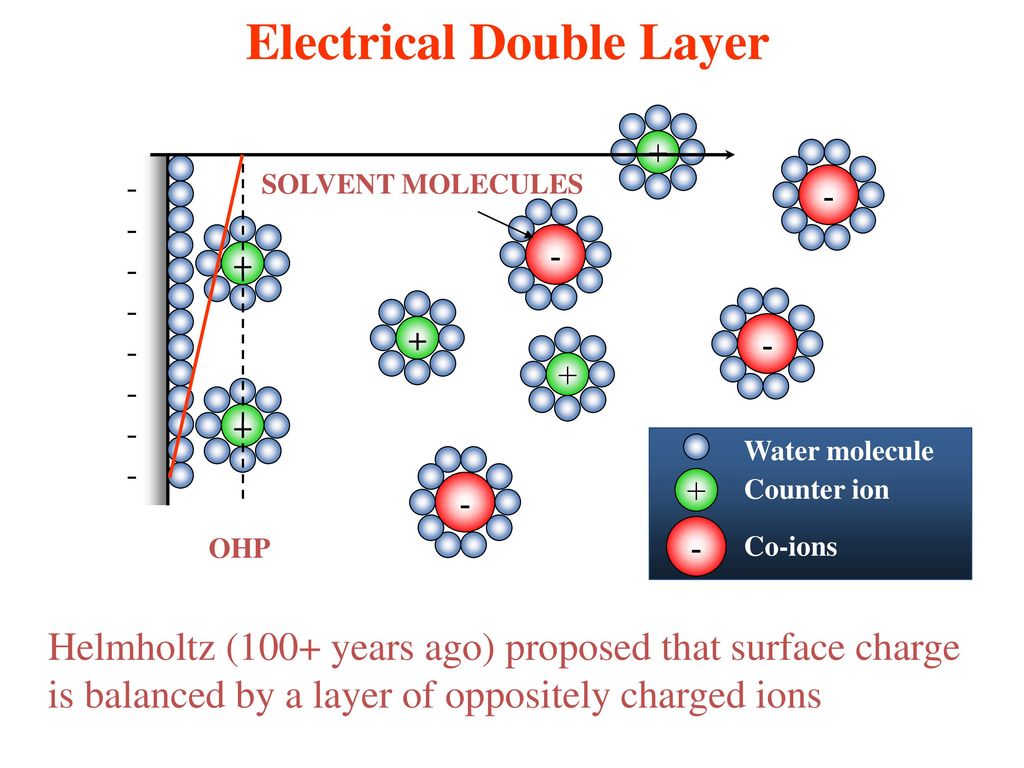
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

Chapter 3 transport phenomena in electrolytic systems and concentration overpotential. - ppt video online download
Spectroscopy of Electrochemical Systems

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields

Fabrication of Ag@Co-Al Layered Double Hydroxides Reinforced poly(o-phenylenediamine) Nanohybrid for Efficient Electrochemical Detection of 4-Nitrophenol, 2,4-Dinitrophenol and Uric acid at Nano Molar Level

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields

PDF) Comparison between Cottrell diffusion and moving boundary models for determination of the chemical diffusion coefficients in ion-insertion electrodes

The Cottrell Experiment and Diffusion Limitation 2/3 - The Cottrell Experiment - PalmSens
The Cottrell Experiment and Diffusion Limitation 3/3

Basic potential step and sweep methods

Electrochemistry with Stationary Disk and Ring−Disk Millielectrodes in Magnetic Fields