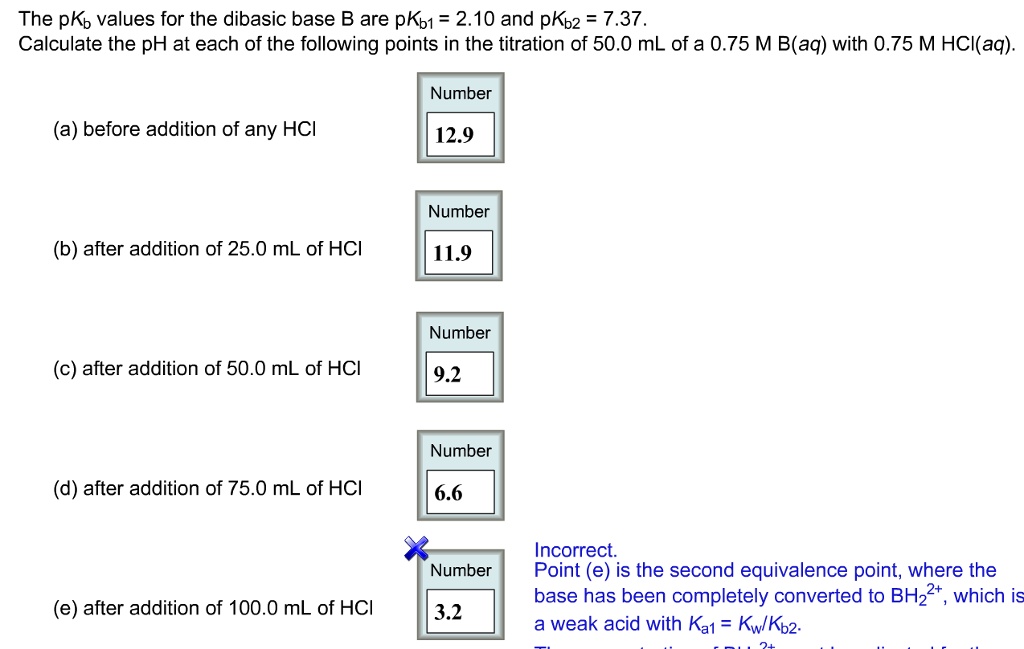
SOLVED: The pKb values for the dibasic base B are pKb1 = 2.10 and pKb2 = 7.37. Calculate the pH at each of the following points in the titration of 50.0 mL
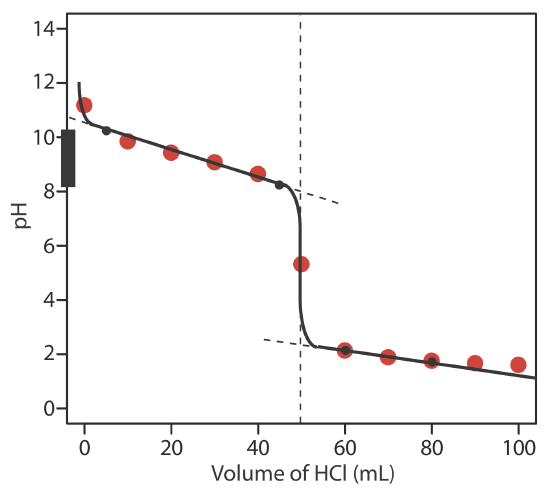
6.6: pH Calculations for Acid–Base Titrations - Chemistry LibreTexts

PH Adjuster and Buffering, PDF, Buffer Solution
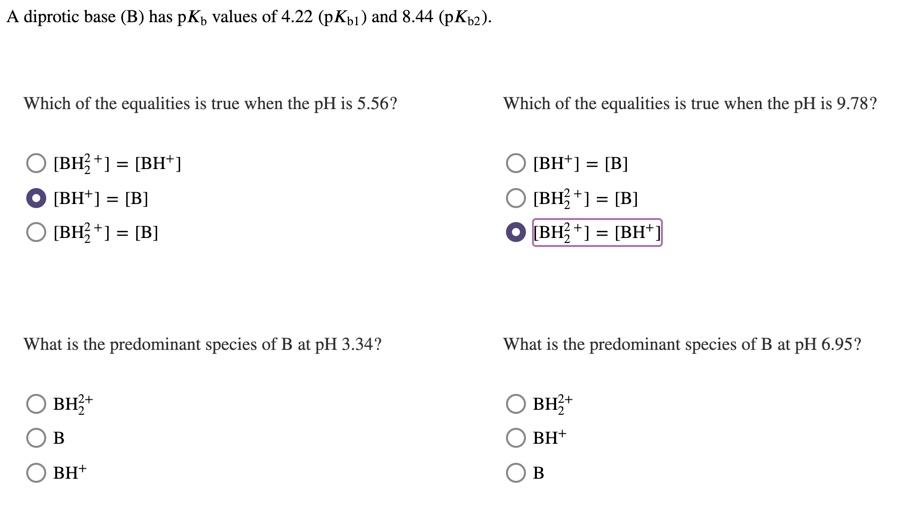
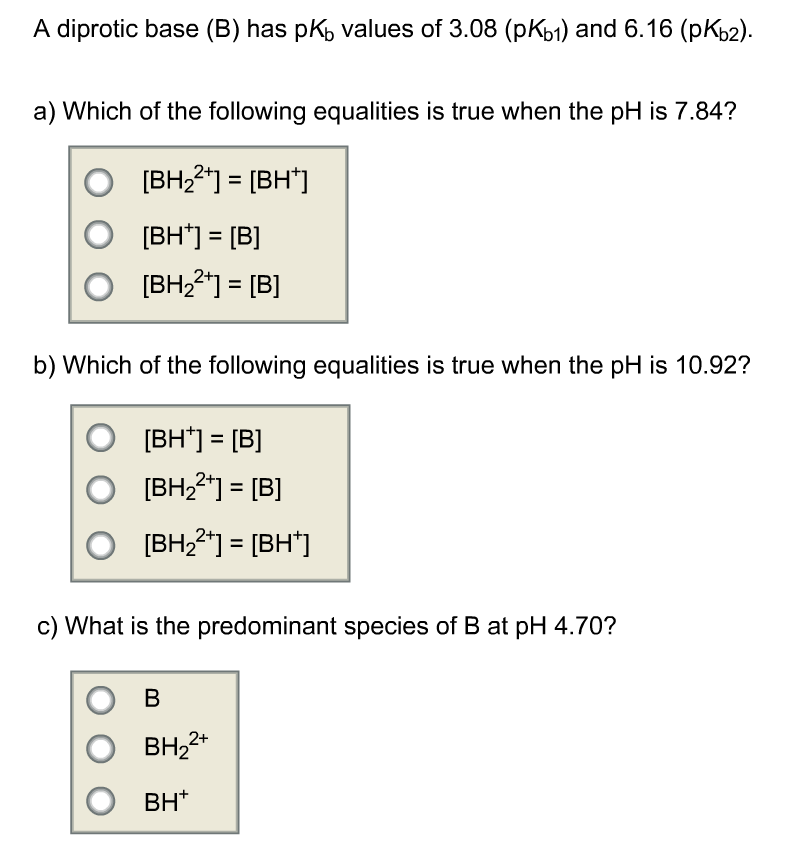
Solved A diprotic base (B) has pK) values of 4.22 (pKb) and

OneClass: The pKb values for the dibasic base B are pKb1 = 2.10 and pKb2 =7.85. Calculate the pH at e

CHEM 14.1-14.6 except 14.5 Flashcards

OneClass: A weak base (B) has a pKb value of 5.77. a) At what pH is [BH ] = [B]? b) What is the predo

Diprotic & Polyprotic Acids
The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points

Harris Quantitative Chemical Analysis 8th edition

The pKb values for the dibasic base B are pKb1=2.10 and pKb2=7.54. Calculate the pH at each of the points