DCTD's Cancer Therapy Evaluation Program Convenes 2019 Early Drug Development Meeting, News & Events
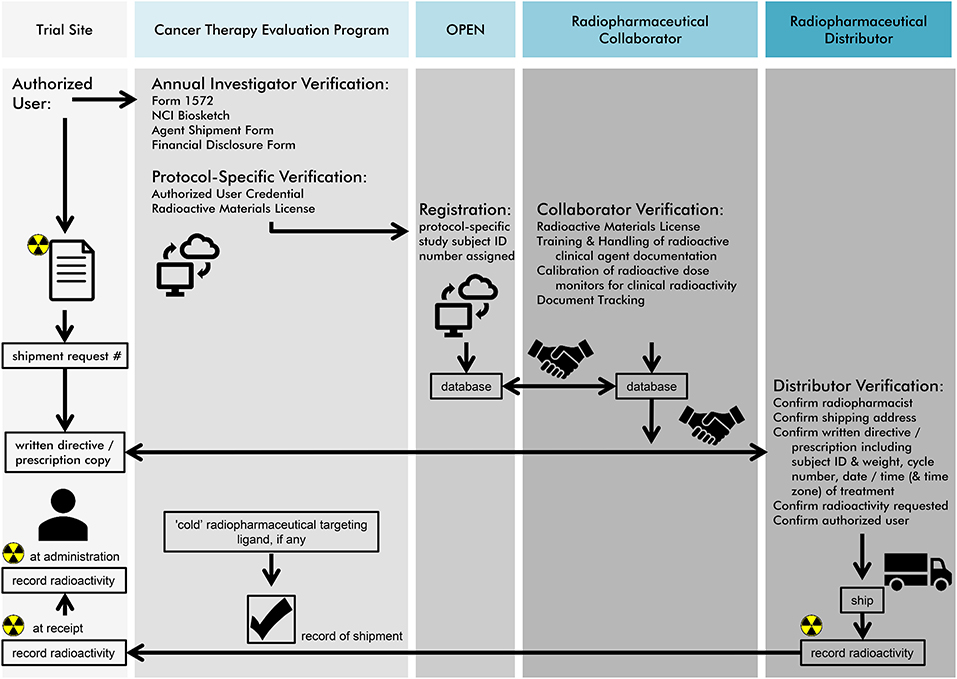
Frontiers Radiopharmaceuticals for Persistent or Recurrent Uterine Cervix Cancer

CTEP Protocol and Information Office (PIO) Overview. - ppt download

FDA Final Rule & Revised CTEP Guidelines for Expedited Reporting of Adverse Events S. Percy Ivy, MD Associate Chief, Senior Investigator Investigational. - ppt download

Branches of the Cancer Therapy Evaluation Program (CTEP) at the
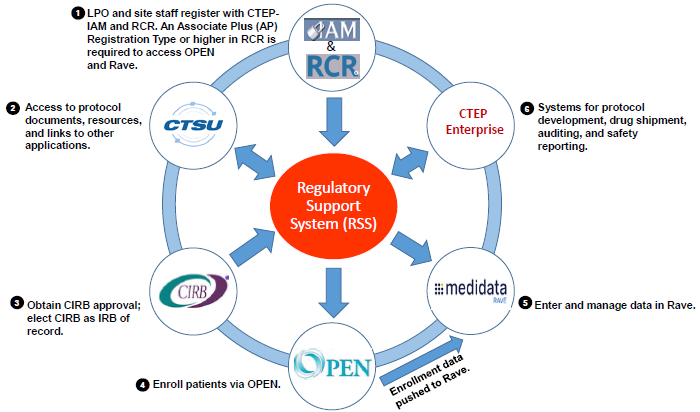
NCI ETCTN Program Infrastructure, Initiatives/Programs

PDF) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment

In Remembrance of Dr. Timmer - Swallowing Cross-System Collaborative

Finding Experts for Clinical Trials - ppt download

ECOG-ACRIN Roster Update Form

Charles Kunos - National Cancer Institute (NCI)

Branches of the Cancer Therapy Evaluation Program (CTEP) at the

In Remembrance of Dr. Timmer - Swallowing Cross-System Collaborative
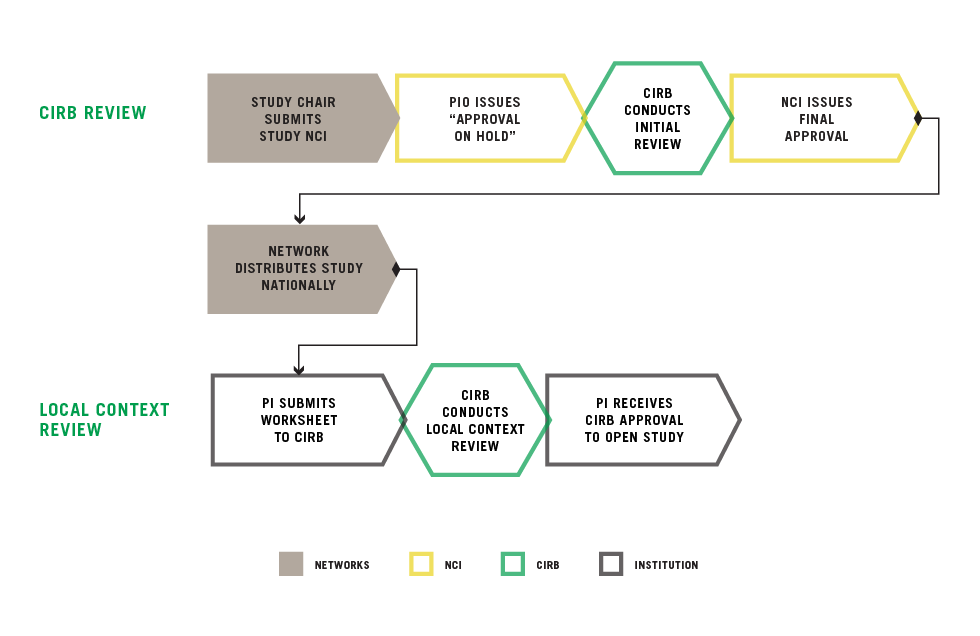
Overview of the Study Review Process
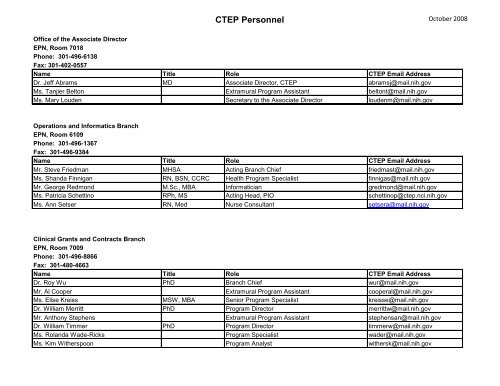
CTEP Personnel - Cancer Therapy Evaluation Program (CTEP)